BioBase® REGISTRY
Technology backed by clinical data

Interested in the BioBase® Registry, studies, and outcomes?
The BioBase® Registry is a multicenter, observational, quality-assessment repository. It is sponsored by Innovasis as part of our commitment to quality and patient outcomes. This program allows surgeons to collect patient outcomes and fusion data in a secure, HIPAA-compliant database that is accessed on a realtime basis.
PATIENT REPORTED MEASURES
• Oswestry disability index (ODI), Neck Disability Index (NDI)
• Visual Analogue Scale (VAS)
• EQ-5D™
• Patient-Reported Outcome Measurement Information System (PROMIS)
• Patient Satisfaction
• Pre-op, surgery and post-op CRFs
SURGEON PORTAL
• Web-based portal, HIPAA-compliant, de-identified data
• Core Lab assessment of fusion rates for HA PEEK at 6 weeks and 3, 6, 12, and 24 months post op.
• Track outcomes against aggregate peer data
• Analyze patient satisfaction and patient reported outcomes
Information Collected
The UNITY platform contains various validated questionnaires, which can be supplemented by individual questionnaires, and can be used on tablets, terminals, or desktop PCs directly by the patient or in interview mode. Data points collected include: Age, Sex, BMI, Co-morbidities, Employment Status, Diagnosis, Treatment, Complications.
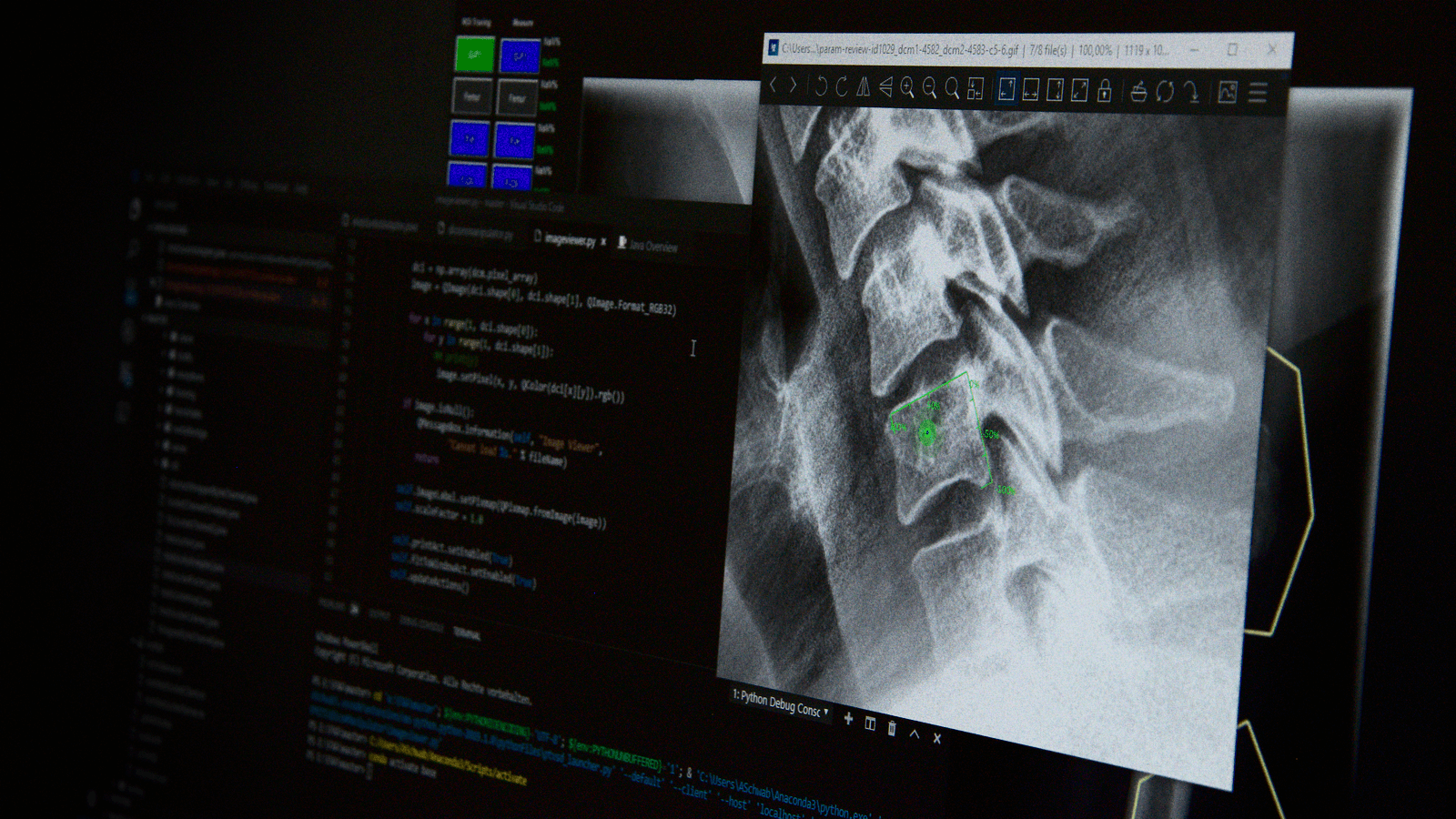
AI Supported Image Assessment
• Absence of lucencies around device
• Absence of graft subsidence or migration
• Segmental ROM
• Absence of translational AP-motion or instability
Contact Us Today
You can apply to participate in the BioBase™ Registry and gain access to valuable tools for yourself, and the patients you serve.
Innovasis staff is available to assist with registry set-up, software training, and database support.
A radiographic core lab, Raylytic provides imaging assessment and database support.
To learn more about eligibility and participation in The Innovasis BioBase™ Data Registry, please contact us

Frequently Asked Questions
Who can participate?
Any spine surgeon or spine group may participate in the Registry. You must be able to use the Innovasis HA PEEK products and have the staff to upload the data into the program.
Are there any costs to participate?
There may be some cost to participate depending on IRB fees. There are no need for additional equipment. You will need to designate a person on your staff to upload the data. Innovasis will underwrite staffing resources needed to administer the registry. There should be no extra cost to the patient as the follow up visits should be consistent with surgical follow up standard of care.
How do I input my patient Data?
The registry is run on a secure web based portal called Unity. Patients may use a tablet, provided by Innovasis, to take Patient Reported Outcome Measures(PROMS) and are electronically stored on a secure server. A member of your staff will be responsible for uploading radiographic images and clinical report forms using the same website.
Is the patient's data secure?
Our program anonymizes PROMS and Radiographs so that data cannot be tracked back to the patient. The data base is HIPAA compliant.
What is the impact of the database?
The database will allow users to track PROMS and fusion rates of patients receiving HA PEEK devices.
Will we have access to outcomes of other registry participants?
Participating sites will have access to their own patients data on an individual and aggregate level. Sites will also have access to the data on an aggregate level (entire population).






